About CARI Guidelines
MISSION
CARI Guidelines mission is to improve the quality of care and outcomes for patients with kidney disease in Australia & New Zealand by facilitating the development and implementation of trustworthy clinical practice guidelines based on the best available evidence
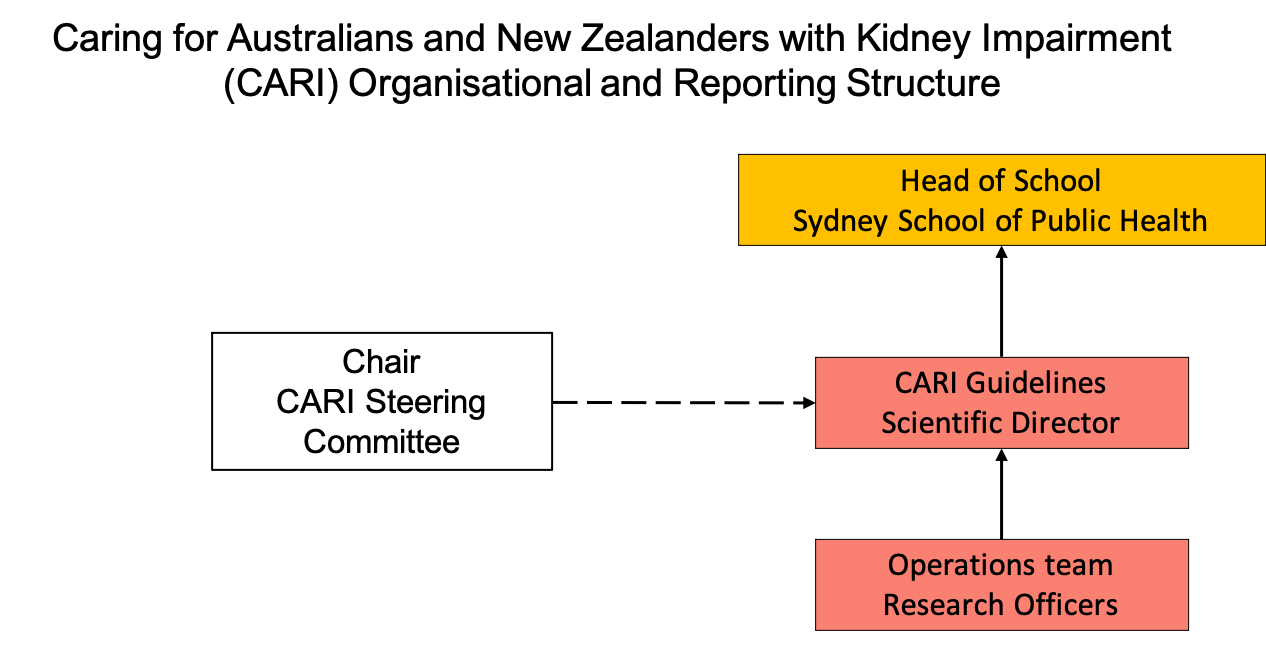
CARI Guidelines Governance
CARI Guidelines operates as an externally funded research program within the School of Public Health, Faculty of Health and Medicine at the University of Sydney. CARI Guidelines day-to-day operations are overseen by a Scientific Director, who reports to the Head of School at the School of Public Health, The University of Sydney. CARI Guidelines Steering Committee provides oversight regarding strategic direction and priority-setting.
Why have guidelines?
Clinical practice guidelines have proved enormously valuable, and adherence to the recommendations translates directly into benefits for patients through improved outcomes, benefits for practitioners through better quality of care, and benefits for providers through improved cost-effectiveness. Guidelines are considered to reduce the use of unnecessary, ineffective, or harmful interventions and to facilitate the treatment of patients with:
Who we are
CARI Guidelines is overseen by Steering Committee of consumers, nephrologists, and allied health professionals. The members serve as an advisory body and provide a strategic direction for CARI Guidelines.
- Vincent Lee, Westmead Hospital, Faculty of Medicine and Health, The University of Sydney, Sydney, NSW (Chair)
- Emily See, University of Melbourne, Melbourne, VIC (Deputy Chair)
- Helen Coolican, PKD Australia, Consumer partner
- Vanessa Cullen, Consumer partner
- Jonathan Craig, Flinders University, Adelaide, SA
- Debbie Fortnum, Sir Charles Gairdner Hospital & University of Western Australia, Perth, WA.
- Min Jun, The George Institute of Global Health, The University of New South Wales, Sydney, NSW.
- Rathika Krishnasamy, University of Queensland, Brisbane, QLD
- Kelly Lambert, University of Wollongong, Wollongong, NSW
- Casey Light, Curtin University, Perth,
- Thu Nyguen, Auckland District Hospital, Auckland
- Carla Scuderi, Royal Brisbane and Women's Hospital, The University of Queensland, Brisbane, QLD
- Andrea Viecelli, University of Queensland, Brisbane, QLD
CARI Guidelines is supported by over a 100 guideline writers, who are selected for their interest and expertise in the clinical area covered by the Guideline Working Groups.
CARI Guidelines is supported by a group of dedicated staff that support guideline Working Groups in the development of guidelines. Staff have expertise in consumer involvement in research and guidelines, evidence synthesis, including the use of GRADE.
- David Tunnicliffe (Scientific Director)
- Mia Abdy
- Brydee Cashmore
- Colm O'Reilly
- Chandana Guha
- Martin Howell
- Allison Jauré
- Ieyesha Roberts
- Nicki-Scholes Robertson
Guideline development process
More than 100 guideline writers have been involved in researching and writing CARI guidelines. Guideline writers are invited to attend a one-day methods workshop run by the CARI office to help equip them for the task of scanning the literature and writing clinical practice guidelines. This training teaches participants how to critically review and summarise the relevant literature on their topic, how to grade the quality of studies and integrate them into their guidelines, and in general, improves their critical appraisal skills.
The CARI office staff assists writers by conducting systematic literature searches, locating relevant trials, and preparing summary evidence tables for each guideline subtopic.
The procedures and methodology used for guideline development and adaptation can be found below.



